Unfortunately, an estimated 24?27% of people living with HIV in the United States are unaware of their status. Furthermore, according to a National Health Interview Survey conducted in 2007, only 36% of adults reported ever being tested for HIV.10
 In hopes of strengthening HIV prevention efforts and maximizing the efficacy of treatment for HIV-positive individuals, the CDC published new recommendations in September 2006 that all adults aged 13-64 should be tested at least once for HIV in a medical setting and that people at higher risk should be tested annually.11 The guidelines recommend opt-out screening, where a patient would be informed that he or she will be tested for HIV infection and given the opportunity to decline, instead of the previously instituted opt-in screening, where written consent was required for testing. The guidelines relaxed requirements on written consent and pre-testing counseling, which can be both time and labor intensive, in the hopes that this would decrease barriers for HIV screening for both patients and providers.
In hopes of strengthening HIV prevention efforts and maximizing the efficacy of treatment for HIV-positive individuals, the CDC published new recommendations in September 2006 that all adults aged 13-64 should be tested at least once for HIV in a medical setting and that people at higher risk should be tested annually.11 The guidelines recommend opt-out screening, where a patient would be informed that he or she will be tested for HIV infection and given the opportunity to decline, instead of the previously instituted opt-in screening, where written consent was required for testing. The guidelines relaxed requirements on written consent and pre-testing counseling, which can be both time and labor intensive, in the hopes that this would decrease barriers for HIV screening for both patients and providers.
 These recommendations have not been fully implemented throughout the United States, since each state has needed to address the guidelines separately. California turned the CDC recommendations into law in 2007 with the passage of AB 682, which waives the need for written consent for HIV testing in this state. Widespread implementation of these recommendations would be especially beneficial to women in this country, given the lack of perceived risk factors for HIV in this population. [Also see "Doctors Urge the Government to Keep up With Medical Progress".]
These recommendations have not been fully implemented throughout the United States, since each state has needed to address the guidelines separately. California turned the CDC recommendations into law in 2007 with the passage of AB 682, which waives the need for written consent for HIV testing in this state. Widespread implementation of these recommendations would be especially beneficial to women in this country, given the lack of perceived risk factors for HIV in this population. [Also see "Doctors Urge the Government to Keep up With Medical Progress".]
HIV Prevention Strategies in Women
Women are biologically and sociologically more vulnerable to HIV infection than men. Women are at greater risk of HIV infection through heterosexual sex than are men simply by virtue of an unequal exchange of genital secretions. The risk of transmission of HIV from a man to a woman is approximately 1 in 1,000 for each sexual contact, whereas the risk of transmission from a woman to a man is much less (approximately one in 2,000).12
Women may also be at increased risk of infection during certain phases of their menstrual cycles (7-10 days following ovulation) because of hormonal interactions with the immune system,13 though further studies are needed on this topic.
Women living in both resource-rich and resource-poor settings are vulnerable to HIV infection through sex for a variety of reasons, often arising from positions of dependence and an inability to insist upon the use of male condoms.
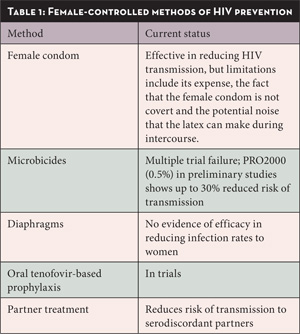
Because women are more vulnerable to HIV through heterosexual sex, prevention efforts need to focus on providing women with female-controlled prevention modalities. Currently, the male condom is the most effective form of HIV prevention for people engaging in sex, followed by male circumcision in terms of specific protection for the male partner. Obviously, women have much less control over the use of a male condom during a heterosexual encounter than men. Women-focused methods for HIV prevention are listed in Table 1. Although the female condom does have some efficacy in reducing HIV transmission, it is not completely covert, as the outer ring or frame is visible outside the vagina. Further reducing its acceptability is the potential noise that the latex can make during intercourse. Additional hindrances to the use of the female condom include the fact that it is not readily available in many countries, can be difficult to insert and remove, can be more expensive than male condoms and historically, can be used only once.
Microbicides and diaphragms have received a lot of attention as they are relatively inexpensive and are completely female-centered modalities of prevention.
Diaphragms were studied with lubricant gel in a large randomized open-label controlled trial of 4,948 HIV-negative Zambian and South African women and showed no efficacy in protecting women against HIV transmission.14
Microbicides are biological or chemical substances that reduce transmission of HIV or other sexually transmitted diseases when applied to the vagina or rectum. Ideally a microbicide would be effective, safe with frequent use, widely available, easy to store and use, surreptitious, and acceptable to a variety of cultures. Most studies to date on different microbicide products, starting with the failure of nonoxynol-9 to protect women against HIV infection (and the possibility of increased transmission rates), have shown disappointing results in terms of interrupting transmission.
A recent study has provided some hope for the field of microbicides and for the possibility of a female-controlled HIV prevention method at last. The interim safety and effectiveness results of a microbicide called PRO2000 (0.5%) were released at the 2009 Conference of Retroviruses and Opportunistic Infections (CROI) in Montreal, Canada a few months ago. PRO2000 (0.5%) is a highly negatively-charged molecule (a polyanionic polymer) that is designed to interfere with HIV entry into target calls. The HPTN035 trial was also designed to study another microbicide product (BufferGel) whose primary action is to lower vaginal pH levels. The trial enrolled 3,909 women from Malawi, South Africa, the United States, Zambia, and Zimbabwe, into four arms: the PRO2000 (0.5%) gel arm, the BufferGel arm, a placebo gel arm, and a no gel arm. The trial followed participants for a mean of 20.4 months.
Both microbicide products showed favorable safety profiles in the trial. While BufferGel did not show a protective effect against HIV, the trial indicated that PRO2000 was at least 30% more effective than any other arm in the study in preventing new HIV infections. This preliminary trial was not designed to generate definitive data on PRO2000, although the data is highly suggestive of the potential efficacy of this product. These findings were viewed with a great deal of enthusiasm in the microbicide and HIV prevention fields and other studies are underway. Studies that are looking at the efficacy of a once-a-day pill (tenofovir or tenofovir/emtricitabine) for HIV-negative people to protect themselves against HIV infection (the pre-exposure prophylaxis or PrEP trials) are also underway.
No comments:
Post a Comment